Physical Chemistry and Electrochemistry

Research projects:
Our research projects include:
- All Copper redox flow battery (CuRen-M)
- Electrodialysis with bipolar membranes (CREDIT). Finished in the end of 2023.
- Electrochemistry at the liquid-liquid interface (ITIES). No more active.
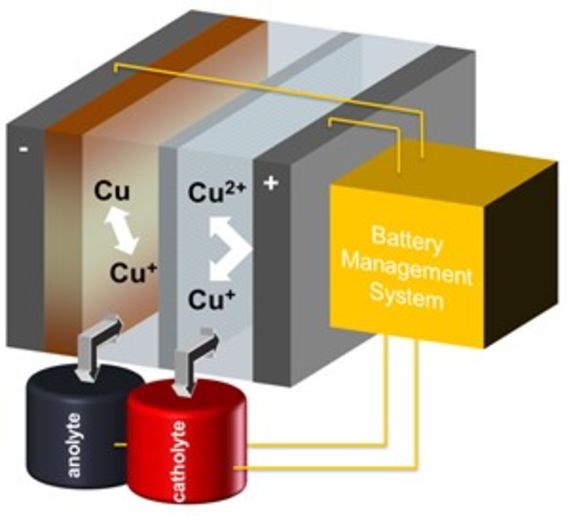
All Copper redox flow battery (CuRen)
The problem of wind and solar energy production is that it is intermittent, not always meeting the need of electric power. Therefore, storing renewable energy to level consumption peaks and lows becomes very important. Large amounts of electric energy are mainly stored in pumped hydropower. From electrochemical methods a flow battery is practically the only one which is easily scalable to the MWh range. The principle of operation of a redox flow battery is presented in Figure 2, also showing the electrode reactions of the all-copper RFB. The capacity of a RFB can in principle be increased without limits by increasing the volume of the electrolyte tanks.
The vanadium RFB is already commercialized but the limited availability of vanadium prevents its use on the global scale. Hence, the focus of our research is to develop a RFB that is based on copper electrochemistry. The study has been funded by EU and Business Finland
Current project is found here.
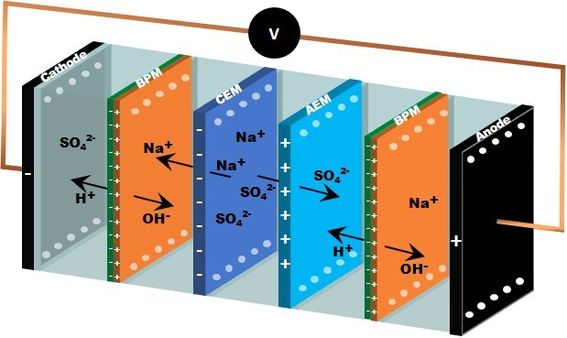
Electrodialysis with bipolar membranes (CREDIT)
In hydrometallurgy, leaching is usually done with sulfuric acid that is neutralized by alkaline, resulting significant amounts of aqueous sulfate salt as waste. The amount of sodium sulfate waste in mines and mineral processes will grow considerably in the future as the need for battery chemicals for energy storage and electric vehicles are continuously increasing. Its low value with high processing costs and the savings in chemical costs are critical issues for its recycling. We are developing a novel technology concept for water treatment and chemical recovery of highly concentrated low-value sulfate waste from a battery chemical plant.
The main aim of the project CREDIT is to scale-up the bipolar electrodialysis (BPED) technology for recycling sodium sulfate in hydrometallurgical processes by converting it to sodium hydroxide and sulfuric acid. We will also provide an economically feasible bipolar membrane-based technology which will reduce environmental discharges. Furthermore, we shall focus on also most suitable pretreatment and post-treatment technologies. The project is financed EIT-Raw Materials program; the other participants are VTT Espoo, Finnish Minerals Group, Norilsk Nickel Harjavalta, and SUEZ Water Technologies.
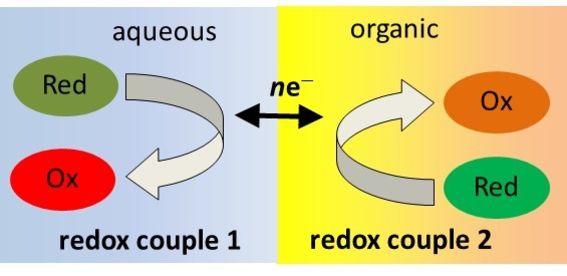
Electrochemistry at the liquid-liquid interface (ITIES)
During the past 30 years, we have studied the electrochemistry of liquid-liquid interfaces, which is quite an original area of research in the field of electrochemistry. Now the research is focused to the metal extraction and reduction boosted with Galvani potential difference across the interface. Experiments are done with the scanning electrochemical microscope (SECM), fixing the Galvani potential difference between the phases via electrolytes distribution (LiTB and BATB).
Funding: Technology Industries of Finland Centennial Foundation


Teaching:
- Electrochemistry
- Transport processes






