Metallurgical Thermodynamics and Modelling

Our research areas:
1. Study of chemical reactions, phase equilibria & elemental distribution in existing and new metallurgical processes:
-
Primary ore feeds become leaner and more complex
-
New feeds (E-scrap, WEEE, recycled batteries
2. Development of Process models, calculational tools & thermochemical databases for pyro- and hydrometallurgical processes
3. Study of large industrial processes on a very detailed level through laboratory studies and modeling, Including non-metallurgical processes (e.g. biomass combustion, waste incineration, high-T corrosion)
The Group is responsible for teaching and research in metallurgical thermodynamics.
The research projects focus on experimental thermodynamics. We utilize experimental techniques, such as equilibration-quenching, EMF measurements, high-temperature furnace treatments to study phase equilibria and thermodynamic properties of metallurgical slags, mattes, and alloys.
We also utilize and develop advanced thermodynamic modeling databases and software for modelling and equilibrium calculations in close cooperation with industrial partners within the field of metallurgical engineering. The industrial projects deal with metal-making processes in the areas of liquid and solid phases (e.g. flash smelting, roasting of sulphides, and salts decomposition by the fluidized bed technology).
The Group is establishing National Centre of Thermodynamics, in cooperation with external partners: we collaborate closely also with universities in Finland, the Nordic countries, South Africa, Canada, China and Indonesia.
Ongoing projects:
Finland-based Circular Ecosystem of Battery Metals, BATCircle
Led by Aalto University, the Finland based circular ecosystem of battery metals consortium (BATCircle in short) aims at improving the manufacturing processes of mining industry, metals industry and battery chemicals, and to increase the recycling of lithium-ion batteries. Its goal is to strengthen the cooperation between companies and research organisations in Finland, and to find new business opportunities.
Symbiosis of metals production and nature, SYMMET
There is currently not enough mining capacity to meet the demand for metals while the use of renewable energy, such as wind and solar energy, and electro-mobility is increasing. The Symbiosis of Metals Production and Nature (SYMMET) project’s objective is to find solutions for the sustainability gap caused by the ever-increasing demand for metals.
FLASH
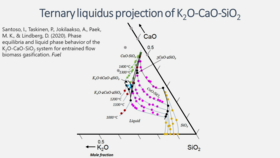
Predicting the FLow behavior of ASH mixtures for production of transport biofuels in the circular economy

Daniel Lindberg, head of the research groupJoin us to study the chemical details for industrial metallurgical processes through advanced experimental, analytical and modeling tools.
Research group members:
Related content:
New Academy Projects to be launched in September
New Academy Projects funded by the Academy of Finland involve expertise from all six Aalto schools

Towards carbon-neutrality in metal production – new €18 million project to develop solutions for reducing CO2 emissions
Metal processing industry has the greatest greenhouse gas reduction potential of Finland's manufacturing industries. Joint project between companies and universities is a significant step towards carbon-neutral metal production.

Creating a unique professional profile by combining different fields of science
Professor Daniel Lindberg, a geologist and a chemist, encourages his students to study combinations of different subjects and fields of science.