Microsystems Technology
The experimental facilities include extensive electrochemical analysis equipment,biocompatibility testing facilities including several different cell lines, different optical and electron microscopes, electrochemical atomic force microscopy and so forth. In addition, extensive utilization of facilities of Micronova and the Nanomicroscopy Center through close collaboration with several groups in Aalto provides the group access to top-class processing and analysis facilities.
Focus on Materials
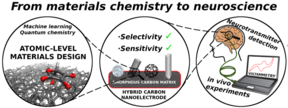
The current focus of the Microsystems technology group is on understanding the physical and chemical properties of materials starting right from the atomic level (Figure 1). The research centers largely on carbon-nanomaterial-based electrochemical biosensors and on multilevel computational studies of their surface properties in various environments. Recently one of our focus areas has been computational deconvolution of spectroscopy results (Figure 2) to obtain detailed local chemical information about nanocarbon surfaces and thus their performance in different applications.
Applications in the biomedical sector
We apply our results from the fundamental scientific investigations to realize designer bioprobes to be used in detection of various biomolecules of interest, such as neurotransmitters dopamine and glutamate as well as of different drug molecules, including paracetamol and opioids, from whole blood samples (Figure 3). These efforts strive towards realization of new groundbreaking analytical tools for neurobiologists as well as of developing new point-of-care (POC) diagnostic devices for clinical and home use.
![Figure 1. Surface roughness and atomic film structure of tetrahedral amorphous carbon deposited at 60 eV, calculated as the mean absolute deviation of surface height from its average. Purple, red, orange, yellow, and blue atoms represent one-, two-, three-, four-, and fivefold coordinated C atoms, respectively [1].](/sites/default/files/styles/o_914w_ah_n_nu/public/2018-11/Fig-1.png?itok=T-hnETZA)
Figure 1. Surface roughness and atomic film structure of tetrahedral amorphous carbon deposited at 60 eV, calculated as the mean absolute deviation of surface height from its average. Purple, red, orange, yellow, and blue atoms represent one-, two-, three-, four-, and fivefold coordinated C atoms, respectively.
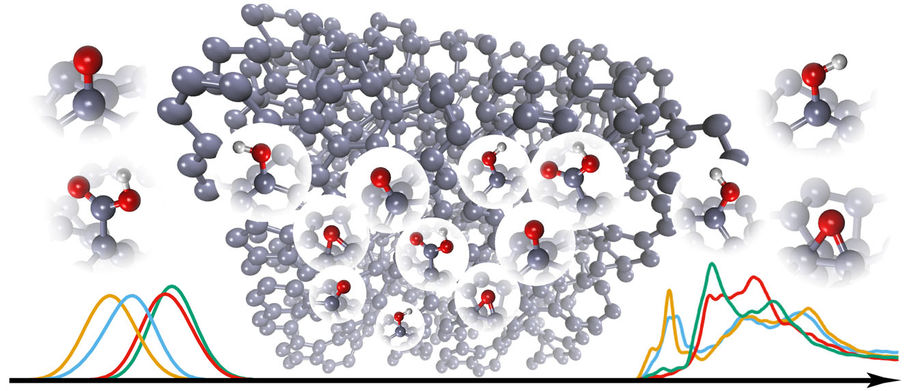
Figure 2. Schematic presentation how the spectroscopy data (in this case from x-ray absorption spectroscopy (XAS) measurements) can be computationally transformed into a atomic level view of the nanocarbon surface structure.
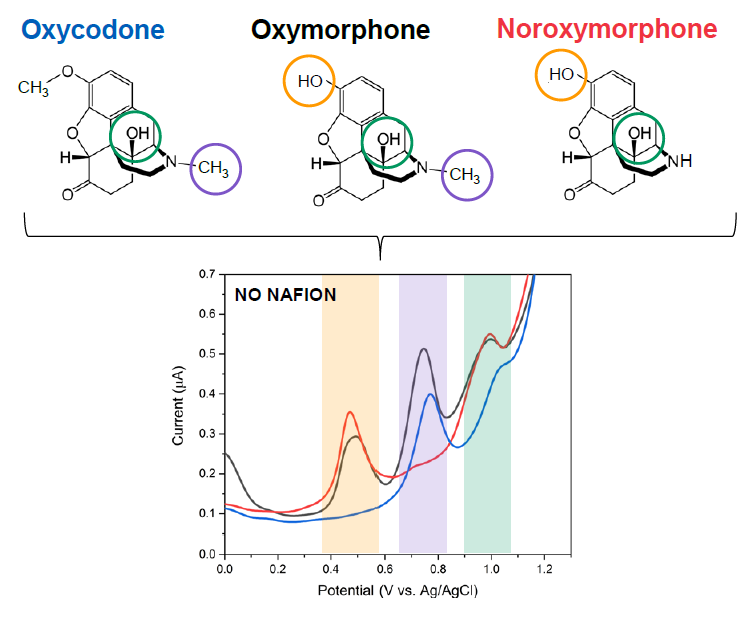
Figure 3. Differential pulse voltammogram (DPV) showing the three oxidation peaks associated with the redox reactions of oxycodone.
Co-operation around the World
The group carries out extensive collaboration with several top Universities around the world. In the field of computational studies, we have a close collaboration with University of Cambridge (UK) and University of Oxford (UK). In the field of advanced spectroscopy methods, we work with Stanford University (US). In the field of electrochemistry, the main collaborators are University College London (UK) and University of Alicante (Spain). Further, nanomaterial growth and characterization benefits from the close cooperation with NASA (Ames Research Center). National collaboration has been especially fruitful with the Neuroscience Center from University of Helsinki, pain clinic at the Hospital district of Helsinki and Uusimaa (HUS), as well as across the different schools in Aalto University. We also collaborate extensively with the medical diagnostics sector in Finland.
Contact
The group is led by Professor Tomi Laurila
Associate Professor, Microsystem technology
Adjunct Professor, Electronics Reliability and Manufacturing
Department of Electrical Engineering and Automation
School of Electrical Engineering and Department of Chemistry and Materials Science
School of Chemical Engineering
Aalto University
Email: Tomi.Laurila@aalto.fi
GSM: +358503414375
Group members

Press Releases
Latest press releases from the Microsystems Technology research group
Researchers can now obtain more accurate information than ever before on the structure and surface chemistry of carbon.
Sensors manufactured with carbon-based materials can provide uniquely accurate and real-time information on hereditary diseases or the concentrations of drugs in the body. In addition to medicine, carbonaceous materials are used in batteries, solar cells and water purification.
Other elements, such as hydrogen and oxygen, are almost always present in carbon-based materials, which alters the materials’ properties. Therefore, modifying materials for desired applications requires atomic-level knowledge on carbon surface structures and their chemistry. Researchers at Aalto University, the University of Cambridge, the University of Oxford and Stanford University have now taken a significant new step forward in describing the atomic nature of carbonaceous materials.
"Understanding X-ray Spectroscopy of Carbonaceous Materials by Combining Experiments, Density Functional Theory, and Machine Learning. Part I: Fingerprint Spectra", Anja Aarva, Volker L. Deringer, Sami Sainio, Tomi Laurila and Miguel A. Caro. Chemistry of Materials, 2019.
Machine learning is increasing the pace of development of customised carbon surfaces with a wide variety of applications.
The potential applications for tailor-made carbon surfaces are wide and include protective coatings, car parts, biomedical coatings and biosensors. Yet for these developments to be realised, detailed atomic level knowledge is still needed on how carbon surfaces are structured and how they can be modified.
Thanks to the development of a new computational model, Postdoctoral Researcher Miguel Caro is spearheading work in this field by researchers at Aalto University, who work in partnership with Professor Gábor Csányi and Dr Volker Deringer from Cambridge University.
"Reactivity of Amorphous Carbon Surfaces: Rationalizing the Role of Structural Motifs in Functionalization Using Machine Learning", Miguel A. Caro, Anja Aarva, Volker L. Deringer, Gábor Csányi and Tomi Laurila. Chemistry of Materials 2018 30 (21), 7446-7455
Customised carbon surfaces can be used in areas such as medical science and water purification.
Researchers at Aalto University and Cambridge University have made a significant breakthrough in computational science by combining atomic-level modelling and machine learning. For the first time, the method has been used to realistically model how an amorphous material is formed at the atomic level: that is, a material that does not have a regular crystalline structure. The approach is expected to have impact on the research of many other materials.
"Growth Mechanism and Origin of High sp3 Content in Tetrahedral Amorphous Carbon", Miguel A. Caro, Volker L. Deringer, Jari Koskinen, Tomi Laurila, and Gábor Csányi. Phys. Rev. Lett. 120, 166101 (2018).
The effects of tramadol, an opioid drug, vary individually. Now, they can be predicted and monitored more accurately than ever before by quick measuring of drug concentrations.
Thanks to the development of a new sensor by researchers at Aalto University, it is now possible, for the first time, to quickly measure the concentration of tramadol, an opioid drug, from a drop of blood. The research has been conducted in cooperation with University of Helsinki and HUS Helsinki University Hospital. The new development represents a significant step forward, as tramadol use, similarly to other opioids, can easily lead to dependency, cause withdrawal symptoms, and even lead to overdose.
In fact, tramadol tops the list as the deadliest opioid. The new sensor can make more individual and effective treatment of pain possible. It may also help to diagnose and start the treatment of poisoning more rapidly.
Tramadol is a mild opioid, and along with codeine, the most-used opioid in Finland, for example, in the management of post-operative acute pain. It is used for chronic pain as well. Tramadol’s opioid effect is based on the metabolism from tramadol into O-desmethyltramadol (ODMT) metabolite in the liver. However, metabolic rates vary individually depending on genetic differences in metabolism of the liver and also possible combined actions of different drugs. Therefore, a dose of tramadol that might be necessary for one person’s pain relief may result in adverse reactions for someone else.
The expected affect of a drug can be examined by determining its concentration in blood. Currently, this is possible only through laborious and time-consuming laboratory testing. This means that, for the most part, calculating the correct dosage with strong analgesics relies on careful starting dose and small alterations in dosages based on patients’ reactions.
"Simultaneous electrochemical detection of tramadol and O-desmethyltramadol with Nafion-coated tetrahedral amorphous carbon electrode", Elsi Mynttinen, Niklas Wester, Tuomas Lilius, Eija Kalso, Jari Koskinen, Tomi Laurila. Electrochimica Acta, https://doi.org/10.1016/j.electacta.2018.10.148
Latest publications from the group:
Designer carbon materials
- Pande I., Sainio S., Sainio J., Liljeström V., Jiang H., and Laurila T., ” Correlation between microstructure and surface chemistry of carbon nanofibers grown using different adhesive layers”, Diamond and Related Materials, 133, 109713, (2023)
- Pande I, Pascual L., Kousar A., Peltola E., Jiang H., and Laurila T., ” Interface matters - Effects of catalyst layer metallurgy on macroscale morphology and electrochemical performance of carbon nanofiber electrodes”, Diamond and Related Materials, 131, 109566, (2023).
- Rantataro S., Parkkinen I., Pande I., Domanskyi A., Airavaara M., Peltola E., and Laurila T., ” Nanoscale Geometry determines Mechanical Biocompatibility of Vertically Aligned Nanofibers”, Acta Biomaterialia, 146, pp. 235-247, (2022)
- Leppänen E., Etula J., Engelhardt P., Sainio S., Jiang H., Mikladal B., Peltonen A., Varjos I., and Laurila T., “Rapid industrial scale synthesis of robust carbon nanotube network electrodes for electroanalysis”, Journal of Electroanalytical Chemistry, 896, pp. 115255, (2021).
- Sainio S., Leppänen E., Mynttinen E., Palomäki T., Wester N., Etula J., Isoaho N., Peltola E., Koehne J.. Meyyappan M., Koskinen J., and Laurila T., ”Integrating Carbon Nanomaterials with Metals for Bio-sensing Applications”, Molecular Neurobiology, 57, (1) pp. 179-190, (2020).
- Palomäki T., Caro M., Wester N., Sainio S., Etula J., Johansson L-S., Han J. G., Koskinen J. and Laurila T., ” Effect of Power Density on the Electrochemical Properties of Undoped Amorphous Carbon (a-C) Thin Films”, Electroanalysis, 31, pp. 1-11, (2019)
- Laurila T., Sainio S. and Caro M., “Hybrid carbon-based nanomaterials for electrochemical detection of biomolecules”, Progress in Materials Science, 88, pp. 499-594, (2017)
- Sainio S., Nordlund, D., Gandhiraman R. P., Jiang H., Koehne J., Koskinen J. Meyyappan M., and Laurila T., “What Nitric Acid Really Does to Carbon Nanofibers?”, Journal of Physical Chemistry C, 120, (29), 22655-22662, (2016).
- Sainio S., Jiang H., Caro M.A., Koehne J., Lopez-Acevedo O, Koskinen J., Meyyappan M., and Laurila T.,“Structural morphology of carbon nanofibers grown on different substrates”, Carbon, 98, 343-351, (2016)
- Laurila T., Sainio S., Jiang H., Koskinen J., Koehne J. and Meyyappan M., “The role of extra carbon source during the pre-annealing stage in the growth of carbon nanofibers”, Carbon, 100, 351-354, (2016)
Electrochemical properties of nanocarbons
- Leppänen E., Gustafsson E., Wester N., Varjos I., Sainio S., and Laurila T., “Geometrical and chemical effects on the electrochemistry of Single-Wall Carbon Nanotube (SWCNT) network electrodes”, Electrochimica Acta, (accepted), (2023)
- Liljeström T., Kontturi K., Durairaj V., Wester N., Tammelin T., Laurila T., and Koskinen J., ”Protein Adsorption and Its Effects on Electroanalytical Performance of Nanocellulose/Carbon Nanotube Composite Electrodes”, Biomacromolecules, 24, pp. 3806–3818, (2023)
- Nekoueian K., Akhoundian M., Wester N., and Laurila T., "An ultra-sensitive dopamine measurement platform based on molecularly imprinted polymer-carbon hybrid nanomaterials for in vitro use", Electrochimica Acta, 445, 142029 (2023). (IF = 7.336)
- Durairaj, V., Liljeström, T., Wester, N., Engelhardt, P., Sainio, S., Wilson, B. P., ... Laurila T. & Koskinen, J., “Role of nanocellulose in tailoring electroanalytical performance of hybrid nanocellulose/multiwalled carbon nanotube electrodes”, Cellulose, 29, pp. 9217-9233, (2022).
- Leppänen E., Akhoundian M., Sainio S., Etula J., Pitkänen O. and Laurila T., ” Structure-property relationships in carbon electrochemistry”, Carbon, 200, pp. 375 – 389, (2022), Pascual L., Pande I., Kousar A., Rantataro S., and Laurila T., “Nanoscale engineering to control mass transfer on carbon-based electrodes”, Electrochemistry Communications, 140, 107328, (2022).
- Pascual L., Pande I., Kousar A., Rantataro S., and Laurila T., “Nanoscale engineering to control mass transfer on carbon-based electrodes”, Electrochemistry Communications, 140, 107328, (2022).
- Etula J., Wester N., Liljeström T., Sainio S., Palomäki T., Arstila K., Sajavaara T., Koskinen J., Caro M.A., and Laurila T., ”What determines the electrochemical properties of nitrogenated amorphous carbon thin films?”, Chemistry of Materials, 33, pp. 6813–6824, (2021)
- Durairaj V., Li P., Liljeström T., Wester N., Etula J., Leppänen I., Ge Y., Kontturi K., Tammelin T., Laurila T., and Koskinen J., “Nanocellulose / Multiwalled Carbon Nanotubes Composites for Electrochemical Applications – Effect of Nanocellulose Dimension and Surface Functionalization”, ACS Applied Nanomaterials, 4, pp. 5842-5853, (2021)
- Leppänen E., Aarva A., Sainio S., Caro M., and Laurila T., ”Connection between the physicochemical characteristics of amorphous carbon thin films and their electrochemical properties”, Journal of Physics: Condensed Materials, 33, 434002, (2021)
- Leppänen, E., Sainio, S., Jiang, H., Mikladal, B., Varjos, I., and Laurila, T., “ Effect of Electrochemical Oxidation on Physicochemical Properties of Fe‐Containing Single‐Walled Carbon Nanotubes. ChemElectroChem, 7, pp.4136-4143, (2020).
- Peltola E., Aarva A., Sainio S., Heikkinen J., Wester N., Jokinen V., Koskinen J., Laurila T., ” Biofouling affects the redox kinetics of outer and inner sphere probes on carbon surfaces drastically differently - implications to biosensing, Physical Chemistry Chemical Physics, 22, pp. 16630-16640, (2020).
- Sainio S., Wester N., Titus C.J., Nordlund D., Lee S-J., Koskinen J., and Laurila T., “In-situ functionalization of tetrahedral amorphous carbon by filtered cathodic arc deposition”, AIP Advances, 9(8), 085325, (2019).
- Palomäki T., Peltola E., Sainio S., Wester N., Koskinen J. and Laurila T., “Unmodified and multi-walled carbon nanotube modified tetrahedral amorphous carbon (ta-C) films as in vivo sensor materials for sensitive and selective detection of dopamine”, Biosensors and Bioelectronics, 118, pp. 23-20, (2018).
- Chumillas S., Palomäki T., Zhang M., Laurila T., Climent V., and Feliu J., “Analysis of Catechol, 4-Methylcatechol and Dopamine electrochemical reactions on different substrate materials and pH conditions”, Electrochimica Acta, 292, pp. 309-321, (2018).
Computational materials science
- Golze D., Hirvensalo M., Hernández-León P., Aarva A., Etula J., Susi T., Rinke P., Laurila T., and Caro M., ”Accurate computational prediction of core-electron binding energies in carbon-based materials: A machine-learning model combining DFT and GW”, Chemistry of Materials, 34, pp. 6240-6254, (2022)
- Aarva A., Sainio S., Deringer V., Caro M., and Laurila T.,”X-ray spectroscopy fingerprints of pristine and functionalized graphene”, Journal of Physical Chemistry C, 125, pp. 18234-1818246, (2021)
- Caro, M. A., Csányi, G., Laurila, T., & Deringer, V. L. ”Machine learning driven simulated deposition of carbon films: From low-density to diamondlike amorphous carbon”, Physical Review B, 102(17), 17420, (2020)
- Aarva A., Deringer V. L., Sainio S., Laurila T., and Caro M., “Understanding X-ray spectroscopy of carbonaceous materials by combining experiments, density functional theory and machine learning. Part I: fingerprint spectra”, Chemistry of Materials, 31, 22, pp. 9243-9255, (2019).
- Aarva A., Deringer V. L., Sainio S., Laurila T., and Caro M., “Understanding X-ray spectroscopy of carbonaceous materials by combining experiments, density functional theory and machine learning. Part II: quantitative fitting of spectra”, Chemistry of Materials, 31, 22, pp. 9256-9267, (2019).
- Caro M., Aarva, A., Deringer, V., Csányi, G., and Laurila T., "Reactivity of amorphous carbon surfaces: rationalizing the role of structural motifs on functionalization using machine learning", Chemistry of Materials, 30, pp. 7446–7455, (2018).
- Deringer V., Caro M., Jana R., Aarva A., Elliott S. Laurila T., Csányi G., and Pastewka L., ”Computational Surface Chemistry of Tetrahedral Amorphous Carbon by Combining Machine Learning and DFT", Chemistry of Materials, 30, pp 7438–7445, (2018).
- Caro M., Deringer V., Koskinen J., Laurila T., and Csanyi G,” Growth mechanism and origin of high sp3 content in tetrahedral amorphous carbon”, Physical Review Letters, 120, 166101, (2018).
- Aarva A., Laurila T., and Caro M., “Doping as a means to probe the potential dependence of dopamine adsorption on carbon-based surfaces: a first-principles study”, Journal of Chemical Physics, 146, (23), 234704, (2017).
- Caro M., Lopez-Acevedo O., and Laurila T., “Redox potentials from ab initio molecular dynamics and explicit entropy calculations: application to transition metals in aqueous solution”, Journal of Chemical Theory and Computation, 13 (8), 3432–3441, (2017).
Biomedical applications of nanocarbons
- Rantataro S., Parkkinen I., Airavaara M., and Laurila T., ” Real-time selective detection of dopamine and serotonin at nanomolar concentration from complex in vitro systems”, Biosensors and Bioelectronics, (in print), (2023),
- Kujala J., Wester N., Lohela T., Kurkela M., Backman J., Mikladal B., Laurila T., Koskinen J., Kalso E., and Lilius T., “Introduction of an electrochemical point-of-care assay for quantitative determination of paracetamol in fingerprick capillary whole blood”, British Journal of Clinical Pharmacology, 1-6, (2023),
- Kousar, A., Pande, I., Ferrer Pascual, L., Peltola, E., Sainio J., and Laurila, T., ” Modulating the Geometry of the Carbon Nanofiber Electrodes Provides the Control over Dopamine Sensor Performance”, Analytical Chemistry, 95, (5), pp. 2983-2991, (2023).
- Verrinder E., Wester N., Leppänen E., Lilius T, Kalso E., Mikladal B., Varjos I., Koskinen J., and Laurila T., ” Electrochemical detection of morphine in untreated human capillary whole blood”, ACS Omega, 6, pp. 11563-11569, (2021).
- Wester N., Mikladal B., Varjos I., Peltonen A., Kalso E., Lilius T., Laurila T., J and Koskinen J., “Disposable Nafion-coated single-walled carbon nanotube test strip for electrochemical quantitative determination of acetaminophen in finger-prick whole blood sample”, Analytical Chemistry, 92, pp. 13017-13024, (2020).
- Mynttinen E., Wester N., Lilius T., Kalso E., Mikladal B., Jiang H., Sainio S., Kauppinen E., Koskinen J and Laurila T., ” Electrochemical detection of oxycodone and its main metabolites with Nafion-coated single-walled carbon nanotube electrodes”, Analytical Chemistry, 92, pp. 8218–8227, (2020)
- Wester N., Mynttinen E., Etula J., Lilius T., Kalso E., Mikladal B., Zhang Q., Jiang H., Sainio S., Nordlund D., Kauppinen E., Laurila T., J and Koskinen J., “Single-Walled Carbon Nanotube Network Electrodes for the Detection of Fentanyl Citrate”, ACS Applied Nanomaterials, 3, 2, pp. 1203-1212, (2020)
- Wester N., Mynttinen E., Etula J., Lilius T., Kalso E., Kauppinen E.I., Laurila T., and Koskinen J., “Simultaneous detection of morphine and codeine in the presence of ascorbic acid and uric acid and in human plasma at Nafion-single walled carbon nanotube thin film electrode”, ACS Omega, 4, 18, pp. 17726-17734, (2019).
- Mynttinen E., Wester N., Lilius T., Kalso E., Koskinen J. and Laurila T. “Simultaneous electrochemical detection of tramadol and O-desmethyltramadol with Nafion-coated tetrahedral amorphous carbon electrode”, Electrochimica Acta, 295, pp. 347-353, (2019).
- Isoaho N., Peltola E. Sainio S., Koskinen J. and Laurila T., ” Pt-grown carbon nanofibers for enzymatic glutamate biosensors and assessment of their biocompatibility“, RSC Advances, 8, 35802 - 35812, (2018).
- Wester N., Etula J., Lilius T., Sainio S., Laurila T., and Koskinen J., ” Selective detection of morphine in the presence of paracetamol with anodically pretreated dual layer Ti/tetrahedral amorphous carbon electrodes”, Electrochemistry Communications, 86, pp. 166-170, (2018).
- Isoaho N., Wester N., Peltola E., Johansson L-S., Boronat A., Koskinen J., Feliu J., Climent V. and Laurila T., “Amorphous Carbon Thin Film Electrodes with Intrinsic Pt-gradient for Hydrogen Peroxide Detection”, Electrochimica Acta, 251, 60-70, (2017).
- Wester N., Sainio S., Palomäki T., Nordlund D., Singh V.K., Johansson L-S, Koskinen J. and Laurila T., “Partially reduced graphene oxide (PRGO) modified tetrahedral amorphous carbon (ta C) thin films electrodes as a platform for nanomolar detection of dopamine”, Journal of Physical Chemistry C, 121, (14), 8153–8164, (2017).







