Mikrosysteemitekniikka
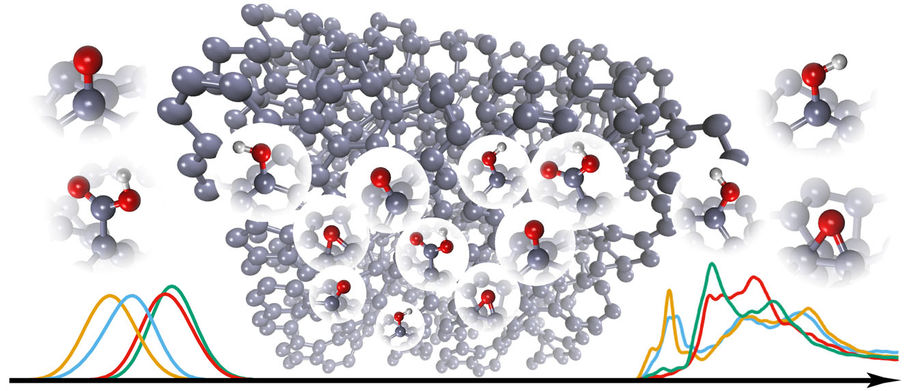
Figure 2. Schematic presentation how the spectroscopy data (in this case from x-ray absorption spectroscopy (XAS) measurements) can be computationally transformed into a atomic level view of the nanocarbon surface structure.
Contact
The group is led by Professor Tomi Laurila
Associate Professor, Microsystem technology
Adjunct Professor, Electronics Reliability and Manufacturing
Department of Electrical Engineering and Automation
School of Electrical Engineering and Department of Chemistry and Materials Science
School of Chemical Engineering
Aalto University
Email: Tomi.Laurila@aalto.fi
GSM: +358503414375
Group members

Press Releases
Latest press releases from the Microsystems Technology research group
Researchers can now obtain more accurate information than ever before on the structure and surface chemistry of carbon.
Sensors manufactured with carbon-based materials can provide uniquely accurate and real-time information on hereditary diseases or the concentrations of drugs in the body. In addition to medicine, carbonaceous materials are used in batteries, solar cells and water purification.
Other elements, such as hydrogen and oxygen, are almost always present in carbon-based materials, which alters the materials’ properties. Therefore, modifying materials for desired applications requires atomic-level knowledge on carbon surface structures and their chemistry. Researchers at Aalto University, the University of Cambridge, the University of Oxford and Stanford University have now taken a significant new step forward in describing the atomic nature of carbonaceous materials.
"Understanding X-ray Spectroscopy of Carbonaceous Materials by Combining Experiments, Density Functional Theory, and Machine Learning. Part I: Fingerprint Spectra", Anja Aarva, Volker L. Deringer, Sami Sainio, Tomi Laurila and Miguel A. Caro. Chemistry of Materials, 2019.
Machine learning is increasing the pace of development of customised carbon surfaces with a wide variety of applications.
The potential applications for tailor-made carbon surfaces are wide and include protective coatings, car parts, biomedical coatings and biosensors. Yet for these developments to be realised, detailed atomic level knowledge is still needed on how carbon surfaces are structured and how they can be modified.
Thanks to the development of a new computational model, Postdoctoral Researcher Miguel Caro is spearheading work in this field by researchers at Aalto University, who work in partnership with Professor Gábor Csányi and Dr Volker Deringer from Cambridge University.
"Reactivity of Amorphous Carbon Surfaces: Rationalizing the Role of Structural Motifs in Functionalization Using Machine Learning", Miguel A. Caro, Anja Aarva, Volker L. Deringer, Gábor Csányi and Tomi Laurila. Chemistry of Materials 2018 30 (21), 7446-7455
Customised carbon surfaces can be used in areas such as medical science and water purification.
Researchers at Aalto University and Cambridge University have made a significant breakthrough in computational science by combining atomic-level modelling and machine learning. For the first time, the method has been used to realistically model how an amorphous material is formed at the atomic level: that is, a material that does not have a regular crystalline structure. The approach is expected to have impact on the research of many other materials.
"Growth Mechanism and Origin of High sp3 Content in Tetrahedral Amorphous Carbon", Miguel A. Caro, Volker L. Deringer, Jari Koskinen, Tomi Laurila, and Gábor Csányi. Phys. Rev. Lett. 120, 166101 (2018).
The effects of tramadol, an opioid drug, vary individually. Now, they can be predicted and monitored more accurately than ever before by quick measuring of drug concentrations.
Thanks to the development of a new sensor by researchers at Aalto University, it is now possible, for the first time, to quickly measure the concentration of tramadol, an opioid drug, from a drop of blood. The research has been conducted in cooperation with University of Helsinki and HUS Helsinki University Hospital. The new development represents a significant step forward, as tramadol use, similarly to other opioids, can easily lead to dependency, cause withdrawal symptoms, and even lead to overdose.
In fact, tramadol tops the list as the deadliest opioid. The new sensor can make more individual and effective treatment of pain possible. It may also help to diagnose and start the treatment of poisoning more rapidly.
Tramadol is a mild opioid, and along with codeine, the most-used opioid in Finland, for example, in the management of post-operative acute pain. It is used for chronic pain as well. Tramadol’s opioid effect is based on the metabolism from tramadol into O-desmethyltramadol (ODMT) metabolite in the liver. However, metabolic rates vary individually depending on genetic differences in metabolism of the liver and also possible combined actions of different drugs. Therefore, a dose of tramadol that might be necessary for one person’s pain relief may result in adverse reactions for someone else.
The expected affect of a drug can be examined by determining its concentration in blood. Currently, this is possible only through laborious and time-consuming laboratory testing. This means that, for the most part, calculating the correct dosage with strong analgesics relies on careful starting dose and small alterations in dosages based on patients’ reactions.
"Simultaneous electrochemical detection of tramadol and O-desmethyltramadol with Nafion-coated tetrahedral amorphous carbon electrode", Elsi Mynttinen, Niklas Wester, Tuomas Lilius, Eija Kalso, Jari Koskinen, Tomi Laurila. Electrochimica Acta, https://doi.org/10.1016/j.electacta.2018.10.148







