Easy access in vitro platform

Our facilities at Micronova include biosafety-level 2 cabinet and two incubators dedicated for mammalian cell cultures. We also have several analysis tools such as fully automatized high-end fluorescence microscopy, plate reader and PCR, enabling most common cell culture analysis methods. We are open for new collaboration on materials in biological applications. For more information, please contact Professor Tomi Laurila (tomi.laurila@aalto.fi) or Samuel Rantataro (samuel.rantataro@aalto.fi).
Research on bioelectric interfaces
Biological environment sets significant challenges for the measurements. Electrode fouling and uncontrolled host response have been proposed to be the main reasons for sensor failure in vivo. The research on bioelectric interfaces focuses on approaches to solve the above–mentioned problems and to outperform the conventional structures. We are developing means to control both the electrode fouling as well as the host response directly with just the electrode material by utilizing structured carbon–based nanomaterials. This approach will maintain the performance level of the electrode, while typical approaches, namely different coatings, result in reduced analyte diffusion and perfusion to implanted sensors.
Biocompatibility
Our research focuses on in vitro microelectrode arrays, implantable brain electrodes, and investigating the interaction of neural cells and carbon nanomaterials. Ideally, a material would support the growth of neural cells and hinder the activation of glial cells. It would consequently improve tissue integration and prevent the glial scar formation. With vertically aligned carbon nanofibers we have achieved this kind of cellular response, however the dimensions of carbon nanomaterials play a critical role concerning cellular biocompatibility. For example, we have shown that nanoscale geometry determines whether focal adhesions will form or not, which has implications on the development of cytoskeleton but the cell morphology also.
Biofouling
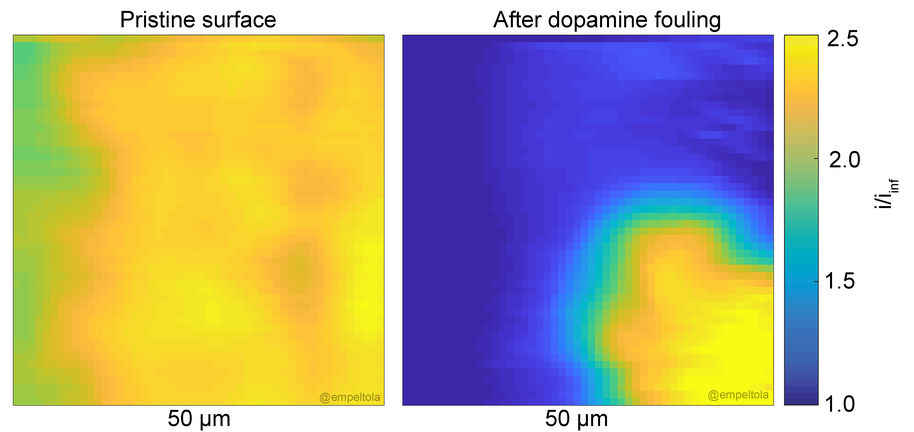
Biofouling is an issue for any sensor in contact with biological fluids. Fouling involves the passivation of the implant by proteins and lipids (and sometimes by the analyte itself). There is need for enhancing our understanding of fouling pathways and the precise mechanism in resistance to fouling to develop more effective and versatile antifouling strategies. Improved strategies towards reduced fouling would benefit all in vitro and in vivo sensor. Currently, it is not unambiguously known how protein adsorption correlates with electrochemical performance of the electrode.
We have developed novel means for investigating electrode fouling by using scanning electron microscopy. This method allows local, real-time, evaluation of the fouling process. It is possible to observe the growth of the fouling layer.
Recent publications
- S. Rantataro, I. Parkkinen, I. Pande, A. Domanskyi, M. Airavaara, E. Peltola, and T. Laurila. Nanoscale geometry determines mechanical biocompatibility of vertically aligned nanofibers. Acta Biomaterialia, 146:235-247, 2022. DOI: 10.1016/j.actbio.2022.04.032
- N. Isoaho, E. Peltola, S. Sainio, J. Koskinen, and T. Laurila. Pt-grown carbon nanofibers for enzymatic glutamate biosensors and assessment of their biocompatibility. RSC Advances, 8:35802–35812, 2018. DOI: 10.1039/c8ra01703d
- T. Palomäki, E. Peltola, S. Sainio, N. Wester, O. Pitkänen, K. Kordas, J. Koskinen, and T. Laurila. Unmodified and multi-walled carbon nanotube modified tetrahedral amorphous carbon (ta-c) films as in vivo sensor materials for sensitive and selective detection of dopamine. Biosensors and Bioelectronics, 118:23–30, 2018. DOI: 10.1016/j.bios.2018.07.018
- N. Isoaho, S. Sainio, N. Wester, L.S. Johansson, E. Peltola, V. Climent, J. Feliu, J. Koskinen, and T. Laurila. Pt-grown carbon nanofibers for detection of hydrogen peroxide. RSC Advances, 8:12742–12751, 2018. DOI: 10.1039/C8RA07766E
- E. Peltola, S. Sainio, K.B. Holt, T. Palomäki, J. Koskinen, and T. Laurila. Electrochemical fouling of dopamine and recovery of carbon electrodes. Analytical Chemistry, 90(2):1408–1416, 2018. DOI: 10.1021/acs.analchem.7b04793
- E. Peltola, J. Heikkinen, K. Sovanto, S. Sainio, A. Aarva, S. Franssila, V. Jokinen, and T. Laurila. SU-8 based pyrolytic carbon for the electrochemical detection of dopamine. Journal of Materials Chemistry B, 5:9033–9044, 2017. DOI: 10.1039/C7TB02469J
- N. Isoaho, N. Wester, E. Peltola, L.-S. Johansson, A. Boronat, J. Koskinen, J. Feliu, V. Climent, and T. Laurila. Amorphous carbon thin film electrodes with intrinsic Pt-gradient for hydrogen peroxide detection. Electrochimica acta, 251:60–70, 2017. DOI: 10.1016/j.electacta.2017.08.110
- N. Isoaho, E. Peltola, S. Sainio, N. Wester, V. Protopopova, B. Wilson, J. Koskinen, and T. Laurila. Carbon nanostructure based platform for enzymatic glutamate biosensors. The Journal of Physical Chemistry C, 121:4618–4626, 2017. DOI: 10.1021/acs.jpcc.6b10612






