Pavlov's classical conditioning inspires materials scientists

Researchers have successfully trained a material to respond to an originally neutral stimulus, a gel that can be taught to melt without needing heating. Their work, recently published in Nature Communications, was inspired by the concept of classical conditioning in behavioural psychology, better known as Pavlov’s dog experiment.
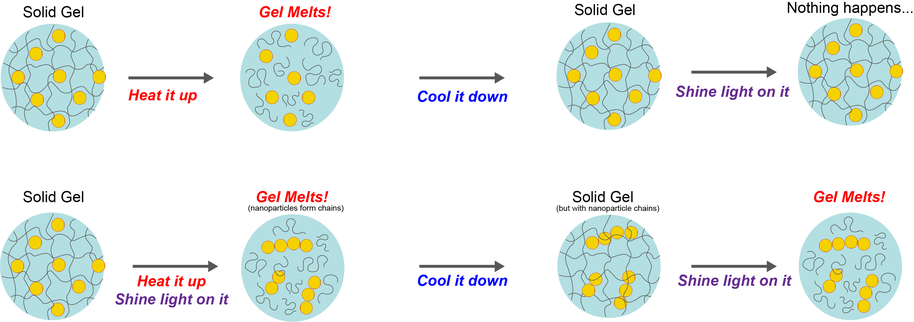
To explore whether classical conditioning could be achieved in artificial materials, the researchers made a solid gel based on agarose, a substance commonly extracted from seaweed, mixed with water and modified gold nanoparticles. When this gel has red and blue light shone on it, nothing happens. If you melt the gel by heating, cool it back down so it solidifies again, and then shine red and blue light on it, nothing exciting happens either. But if you melt the gel while illuminating it with red and blue light, then cool it back down into a gel, the gel will spontaneously melt the next time you shine red and blue light on it. It has thus "learned" to respond to a new stimulus.
In Ivan Pavlov’s famous classical conditioning experiment in experimental psychology dealing with simple forms of learning, a dog could be trained to salivate when it heard a bell. Pavlov trained the dog to behave like this by ringing a bell every time he fed the dog – the dog associated the sound of the bell with its food and would begin to drool when it heard the bell ring. The gel developed by teams from Aalto University and Tampere University mimics this process with the heating corresponding to the food and the coloured light corresponding to the bell.
'Conceptually this is very new, there is not really anyone making materials that show this Pavlovian response. We were interested in introducing the elemental concepts of learning to artificial materials.' explained Dr. Hang Zhang, the postdoc who developed the material and who is the first author on July’s paper in Nature Communications. As a member of the Molecular Materials research group and the HYBER Centre of Excellence at Aalto, he works on biologically inspired materials. Besides Dr. Hang Zhang, the research team included Dr. Hao Zeng and Prof. Arri Priimägi from Tampere University, and Prof. Olli Ikkala from Aalto University. The project was supported by two European Research Council (ERC) funded projects, PHOTOTUNE and DRIVEN.
The gel can be trained because the gold nanoparticles in the mixture are sensitive to the acidity of their surroundings. The nanoparticles are initially spread at random throughout the gel. If you melt the gel and solidify it without illumination, they remain randomly distributed. However, if you melt the gel while illuminating it with blue and red light, the nanoparticles stick together and form little chains. This occurs thanks to a photoacid, a final “secret” ingredient in the gel. The photoacid makes the gel more acidic when the coloured light is shone on it, and when this happens in a melted gel, it causes the nanoparticles to form into chains. When you shine blue and red light on the gel containing chains of gold nanoparticles, the chains heat up in a way that individual nanoparticles themselves do not, due a process called plasmonic coupling. This forms a "triggerable optical memory". Making the chains heat up using blue and red light causes the gel to melt itself.
The gel can later also be made to “forget” this training, paralleling the way in which learning can be forgotten by humans. The trick is to use a combination of chemicals (urea and urease) added to the gel during fabrication, which slowly releases ammonia that breaks up the nanoparticle chains. About 12 hours after training, the material no longer melts when illuminated with light and thus loses its memory.
'In terms of practical applications, there is a long way to go for such a conceptually new project.' Dr Zhang laughed. 'The important thing is that we can condition artificial materials in a programmed way using external stimuli and play with its memory chemically. We foresee that other types of learning materials can be designed with a wide array of induced properties, and that conditioning could become a general concept in materials science.'
Read the full article here: https://doi.org/10.1038/s41467-019-11260-3
Read more news

Environmental Structure of the Year 2025 Award goes to Kalasatama-Pasila tramway
The award is given in recognition of meritorious design and implementation of the built environment. Experts from Aalto University developed sustainability solutions for the project.
Five things everyone should know about creativity
Creativity is not the preserve of artists or a rare innate talent but a human capacity we all share – and one that can be measured, developed, and led for. The two-year Creative Leap project explored how creativity shows up in everyday life and work and how it connects to companies’ financial results. Here are five key takeaways.
Everyday choices: Frank Martela, should we take happiness seriously?
Insights from an assistant professor and philosopher who studies human well-being and motivation.






