Surface Science
Group leader
Dr. Jouko Lahtinen
Research is carried out in the following areas:
- Structural and chemical characterization of nanomaterials
- Interactions and structures of adsorbate species on metal surfaces
- Surface and near surface characterization of oxide materials, nanoparticles and ‘real’ catalysts.
Research
The Surface Science group studies:
- Growth and characterisation of 2D materials
- Interactions and structures of adsorbate species on metal surfaces
- Surface and near surface characterization of oxide materials, nanoparticles and ‘real’ catalysts.
Graphene and other 2D nanostructures
Graphene is a one atomic thick sheet of carbon atoms featuring a honeycomb structure. It has several interesting properties both mechanically and electronically.
These studies have been performed in close collaboration with the Atomic Scale Physics group. The adjacent image shows the moire structure of single layer of graphene on Ir(111) surface studied with LEED I(V) and AFM measurements that yield the local surface topography with pm accuracy.
Ordered structures of adsorbed molecules on single crystal surfaces
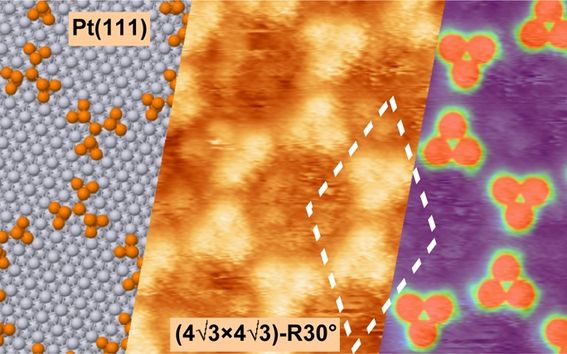
With these studies we aim to increase the understanding of catalytic systems. Adsorption of CO has been studied on metal surfaces and known catalytic promoters and poisons hase been added to change the adsorption behaviour and structure. Typically the system has been studied with XPS and LEED I(V) measurements to give chemical and structural information. The adjacent image shows the adsorption structure of clusters consisting of 14 P-atoms on Pt(111) surface.
Surface characterisation with ESCA
Electron spectroscopy for chemical analysis (ESCA, XPS) is a standard tool for studying the chemical composition of the first few atomic layers of solid material. We have used the method to study a large variety of samples from our collaborators; these include e.g. car exhaust catalysts from Environmental and Chemical Engineering at Oulu University, carbon nano structures from e.g. NanoMaterials group, light emitting silica particles, etc.
Facilities
The Surface Science research group has three multi-technique ultra-high vacuum (UHV) systems located in Nanotalo.
Kratos Axis Ultra ESCA system
The system is an X-ray Photoemission Spectrometer (XPS, ESCA) enabling elemental concentrations,chemical state identification and chemical state mapping of the surface. The system contains a dual anode (Mg and Al Kα source) and a monochromated Al Kα source. The analysis area varies from 110 μm down to 15 μm, and he ultimate lateral resolution is 5 μm. There is also an He-source enabling Ultraviolet Photoemission Spectroscopy (UPS).
Ar Gas Cluster Ion Source (GCIS) capable of generating Ar cluster size up to 2000 atoms. The cluster source enables depth profiling of both hard and soft materials. The ion source also enables Low Energy Ion Scattering Spectrocopy (LEISS).
STM & XPS
The system is a self-combined collection consisting of
- Surface Science SSX-100 electron energy analyzer and monochromatic X-ray source
- Omicron VT SPM variable temperature scanning tunneling microscope
- SPECTALEED reverse view LEED-optics for low energy electron diffraction (LEED)
- evaporation systems for sample preparation in vacuum.
LEED & PM-IRRAS
This is another self-combined system consisting of
- Perkin Elmer PHI 3057 XPS system with a dual anode (Mg and Al Kα) X-ray source and an electron energy analyzer.
- Princeton Research Instruments reverse view LEED-optics
- Bruker Polarization Modulated Ifrared Absorption Spectroscopy (PM-IRRAS)






