Materials for electrochemical energy applications
In the NMG group, we investigate and design novel and low-cost electrocatalysts for catalyzing various electrocatalytic reactions of importance for fuel cells, hydrogen production, and other environmentally important catalytic reactions.
The development of efficient and low-cost electrocatalysts for electrochemical energy devices such as fuel cells and water eletrolyzers plays an essential role because the catalyst determines not only the overall reaction and the device efficiency but also the cost. In NMG group, we investigate and design novel and low-cost electrocatalysts for catalyzing various electrocatalytic reactions of importance for fuel cells, hydrogen production, and other environmentally important catalytic reactions, such as oxygen reduction reaction (ORR), hydrogen oxidation reaction (HOR), hydrogen evolution reaction (HER), oxygen evolution reaction (OER), carbon monoxide oxidation and CO2 reduction reactions.
Carbon nanotubes (CNTs) are known for their exceptional properties in various applications and CNTs have been investigated in NMG for a long time. In NMG we have further shown that CNTs can be utilized to create highly active and durable electrocatalysts when they are modified by metals or heteroatoms such as nitrogen or functionalized with polymers and organometallic compounds. Some of the recent projects in this field are as follows:

Development of novel nanomaterials for catalysis through a novel CVD synthesis method for the growth of carbon encapsulated transition metal nanoparticles (CEMNs) decorated on carbon nanotubes (CNTs).
Journal Reference:
M. Tavakkoli, T. Kallio, O. Reynaud, A.G. Nasibulin, Ch. Johans, J. Sainio, H. Jiang, E.I. Kauppinen, and K. Laasonen, Single-Shell Carbon-Encapsulated Iron Nanoparticles: Synthesis and High Electrocatalytic Activity for Hydrogen Evolution Reaction, Angew. Chem. Int. Ed., 54 (2015) 4535 –4538.
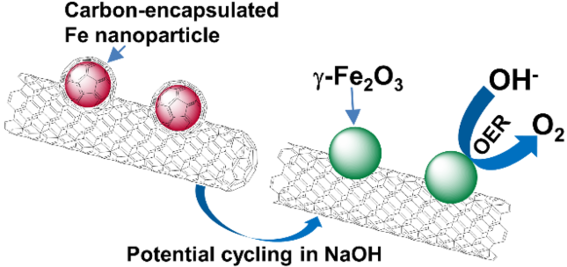
Novel electrochemical modification of transition metal nanoparticles, and carbon nanomaterials for synthesizing active catalysts and efficient materials for high-performance lithium ion batteries.
Journal Reference:
M. Tavakkoli, T. Kallio, O. Reynaud, A.G. Nasibulin, J. Sainio, H. Jiang, E.I. Kauppinen, and K. Laasonen, Maghemite nanoparticles decorated on carbon nanotubes as efficient electrocatalysts for the oxygen evolution reaction, J. Mater. Chem. A, 4 (2016), 5216-5222.
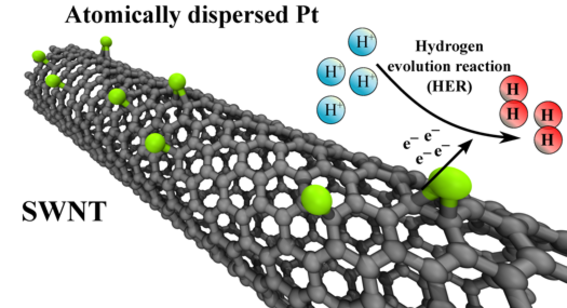
Novel synthesis of pseudo atomic-scale Pt catalysts materials decorated on carbon nanotubes for catalytic applications.
Journal Reference:
M. Tavakkoli, N. Homberg, R. Kronberg, H. Jiang, J. Sainio, E.I. Kauppinen, T. Kallio, and K. Laasonen, Electrochemical Activation of Single-Walled Carbon Nanotubes with Pseudo-Atomic-Scale Platinum for the Hydrogen Evolution Reaction, ACS catalysis, 7 (2017), 3121–3130.
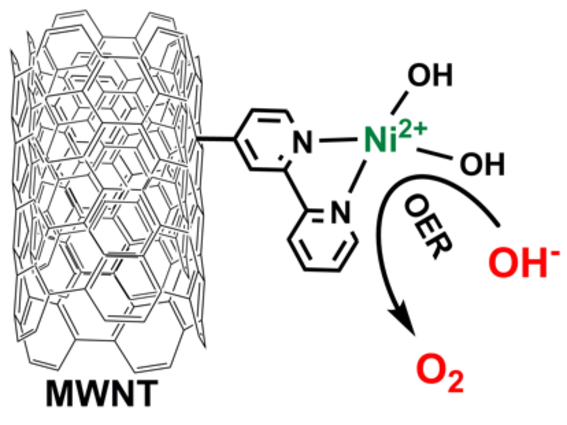
Novel Covalent functionalization of carbon nanotubes with transition metal bipyridyl complexes as a new class of highly active and durable catalyst materials.
Journal Reference:
M. Tavakkoli, M. Nosek, J. Sainio, F. Davodi, T. Kallio, P. Joensuu, P. Joenssu, and K. Laasonen, Functionalized Carbon Nanotubes with Ni(II) Bipyridine Complexes as Efficient Catalysts for the Alkaline Oxygen Evolution Reaction, ACS Catal., 7 (2017), 8033–8041.
Collaborations
The modeling and computational studies of the electrocatalysts designed in NMG are done in the Computational Chemistry group led by Prof. Kari Laasonen. We also work with various groups worldwide in the field of catalysis and carbon nanotubes.
Further information
Dr. Mohammad Tavakkoli
tel. +358 50 4140950
Prof. Esko Kauppinen
tel. +358 40 509 8064
Fax: +358 9 2451 3517






